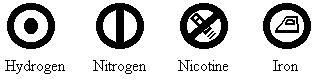As noted recently, rather too many years ago I wrote a thing called ‘THE COMPLETE HISTORY OF SCIENCE: REVISED, UPDATED AND GENERALLY REFURBISHED’ and it saw the light of day, so to speak, in The Mentor, an Australian fanzine. Below is part 4. Part 2 is here. Part 3 I leave to the imagination.
THE COMPLETE HISTORY OF SCIENCE: REVISED, UPDATED AND GENERALLY REFURBISHED.
Part IIII (also known as Part IV)
Isaac Newton: The year Galileo died, Isaac Newton was born. A year later, he had his first birthday, establishing a pattern which was to remain with him all his life. Isaac Newton is best known for three things: Inventing calculus, making up his three laws (thus revealing Kepler’s influence), and inventing apples. He therefore opened a rift between science and religion, as the Church insisted that the apple had been invented by the snake – a dubious proposition indeed.
The discovery of the apple was of considerable gravity. Newton thus called it his theory of apple gravity, though this is generally shortened to the theory of gravity, a meaningless contraction. In the theory, the attractive force between two masses is inversely proportional to their separation squared and proportional to whether one of them is ripe or not. Newton’s greatest leap was to say that this ‘apple force’ also acted upon the moon and other celestial bodies, allowing us to conclude that the moon itself is an apple – presumably very old and, therefore, wrinkly and discoloured.
His three laws were all to do with force. The third one (the important one) states that for every action there is an equal and opposite reaction, a principle that rears its head in business and politics as well as physics.
Newton, however, was also an alchemist. This is a black mark against him. When added to all the extra work that high school students have to do because of him, it becomes doubtful whether he was, after all, a benefactor of mankind.
Lastly, Newton set the trend of having a measurement named after him – the Newton, the unit of force. This is nothing like the ‘Nightstick’, which is the unit of ‘Brute Force’.
Edmund Halley: Edmund Halley was a friend of Newton’s. His invention of comets (“hairy stars”) was a breakthrough. So much so that one was named after him – though comet Edmund was never seen again.
Leibniz: Though less famous than Newton, Leibniz was still famous enough to also invent calculus.
After Newton: After a flurry of activity, science slowed down for a while due to a lack of historically notable figures. Britain in particular suffered, since they’d been letting Newton do all the science and he was dead. In America, Benjamin Franklin connected himself to lighting via a kite. Simultaneously, he: 1) Proved that lighting was a form of electricity and; 2) Invented the ‘perm’ hairstyle.
In Europe things soon picked up again. Mathematics was very popular. In particular, a Frenchman called Lagrange formulated the principle of least action. Like Newton’s third law, this also has been co-opted by politicians.
Great strides were made by the chemist John Dalton. He is, however, best known for his chemistry, in which he resurrected the ancient Greek idea of the ‘atom’. He also invented symbols for each kind of atom:
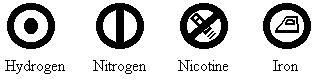 Though his framework has remained largely intact, a number of his identifications have been called into question. And the proposal of naming an element ‘Dolt’ in his honour has floundered.
Though his framework has remained largely intact, a number of his identifications have been called into question. And the proposal of naming an element ‘Dolt’ in his honour has floundered.
But it was good to have atoms, because now people had something to be made of. Also, it would give all those atomic scientists something to do during World War II.
After Dalton came his children. In science also things were picking up. The publication of Frankenstein, by Mary Shelley, gave great impetus to biology (and the odd corpse), and Gregor Mendel (a monk) invented genetics, something which gave all sorts of people excuses for being the sort of people they were.
Faraday & Maxwell etc: Inextricably linked in the history of science are the names Farawell and Maxaday, who between them invented electromagnetism.
There was a lot of quantifying going on. Wellday and Faramax quantified electricity, Mendeleyev quantified the elements, and Arthur Waddington-Smith quantified garden gnomes, though this is sometimes seen as a lesser achievement.
Charles Darwin: No figure stands taller in the annals of nineteenth century science than Charles Darwin, father of evolution. Above all scientists, he really got up God’s nose. His proposal that mankind and the apes had a common ancestor – whom he tentatively dubbed ‘Hubert’ – gave many cause for alarm, all the more when he revealed that a beagle had given him the idea. However, he received quite a bit of support from the wives of boorish men, as these ladies found that his theory dovetailed nicely with their own observations.
Darwin wrote two great books. The Origin of Species, in which he explained evolution, and The Descent of Man, in which he described man’s descent from noble savage to tax collector. Objections came from all directions, generally from people denying that they were related to tax collectors.
Edison: Meanwhile, in America Thomas Edison was busy inventing the twentieth century. He was in a bit of a hurry, since he was working to a deadline. Amongst his inventions were the electric light, the phonograph, and the Spud-O-Matic, though the latter never really took off.
So at the End of the Nineteenth Century: So at the end of the nineteenth century, they thought they knew it all. Newton had worked out Newton’s Laws, Maxwell had worked out Faraday’s Equations, Mendel had worked out Mendel’s Rules, Darwin had worked out Darwin’s Theory, and Alfred Nobel was making things blow up and giving out prizes.
But then the Universe started obeying quantum mechanics, and all that went out the window.